Mole Concept Combustion Questions
Here’s an “ingelious” way to solve combustion questions related to mole concept using model drawings (yep, like in primary school). Let’s look at a typical JC A Level H2 Chemistry question:
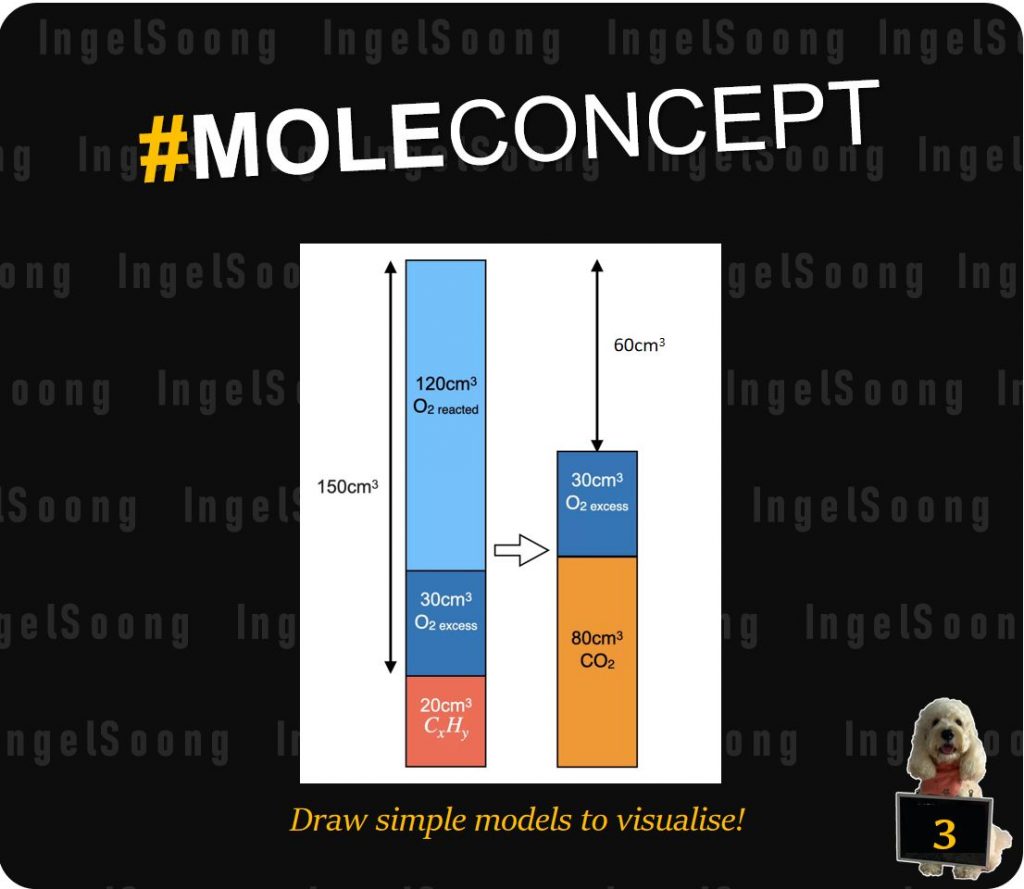
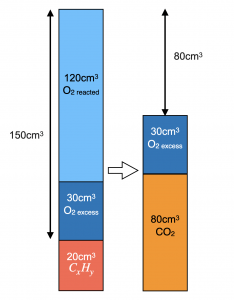
When 20 cm3 of a gaseous hydrocarbon were sparked with 150 cm3 of oxygen and the residual gases cooled to room temperature, a contraction of 60 cm3 occurred. A further contraction of 80cm3 took place when the residual gases were subjected to aqueous NaOH. Find formula of the hydrocarbon. [All gaseous volumes are measured under identical conditions.]
Proposed solution:
CxHy + (x + O2
CO2 + H2O
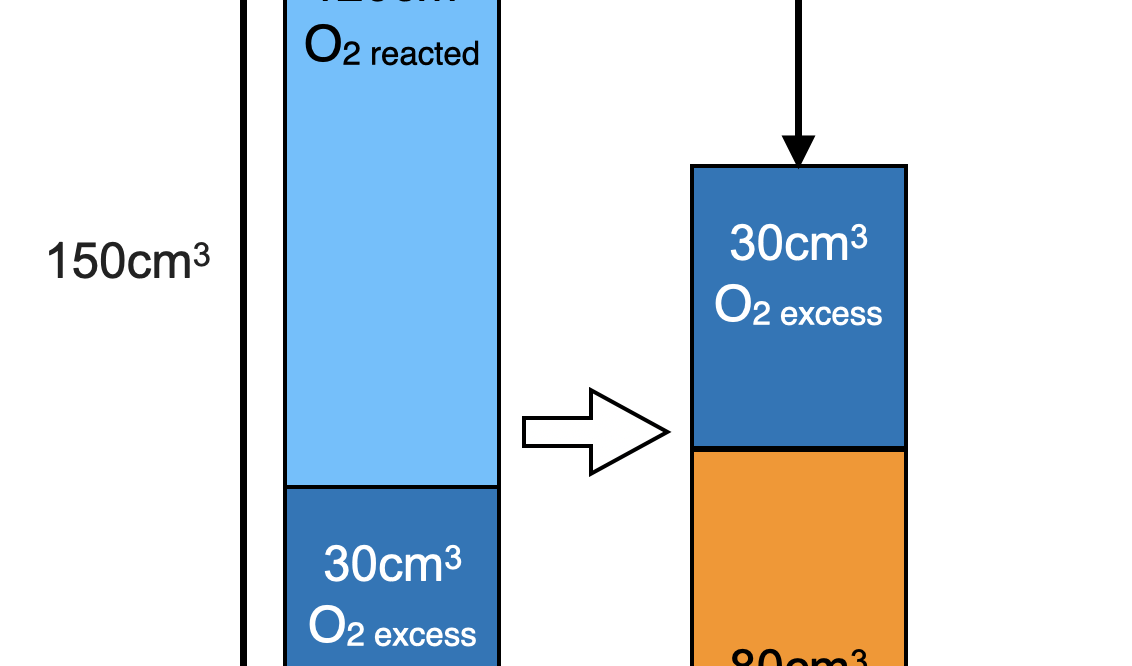
V(CO2) produced = 80 cm3 since it is removed by alkaline NaOH.
Total initial volume before combustion = 150 + 20 = 170 cm3
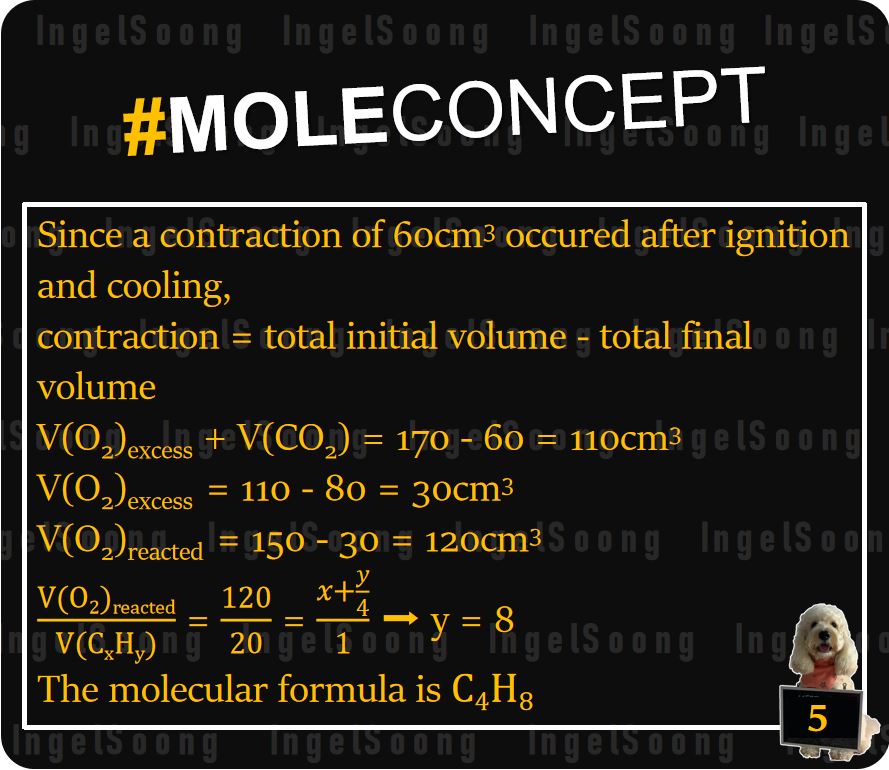
Since a contraction of 60cm3 occurred after ignition and cooling, contraction = total initial volume – total final volume
V(O2) excess + V(CO2) = 170 – 60 = 110 cm3.
V(O2) excess = 110 – 80 = 30 cm3
V(O2) reacted = 150 – 30 = 120 cm3
y = 8
The molecular formula is C4H8.