Chemical Energetics Gibbs Free Energy
Here’s an “ingelious” way to understand Gibbs Free Energy related to chemical energetics.
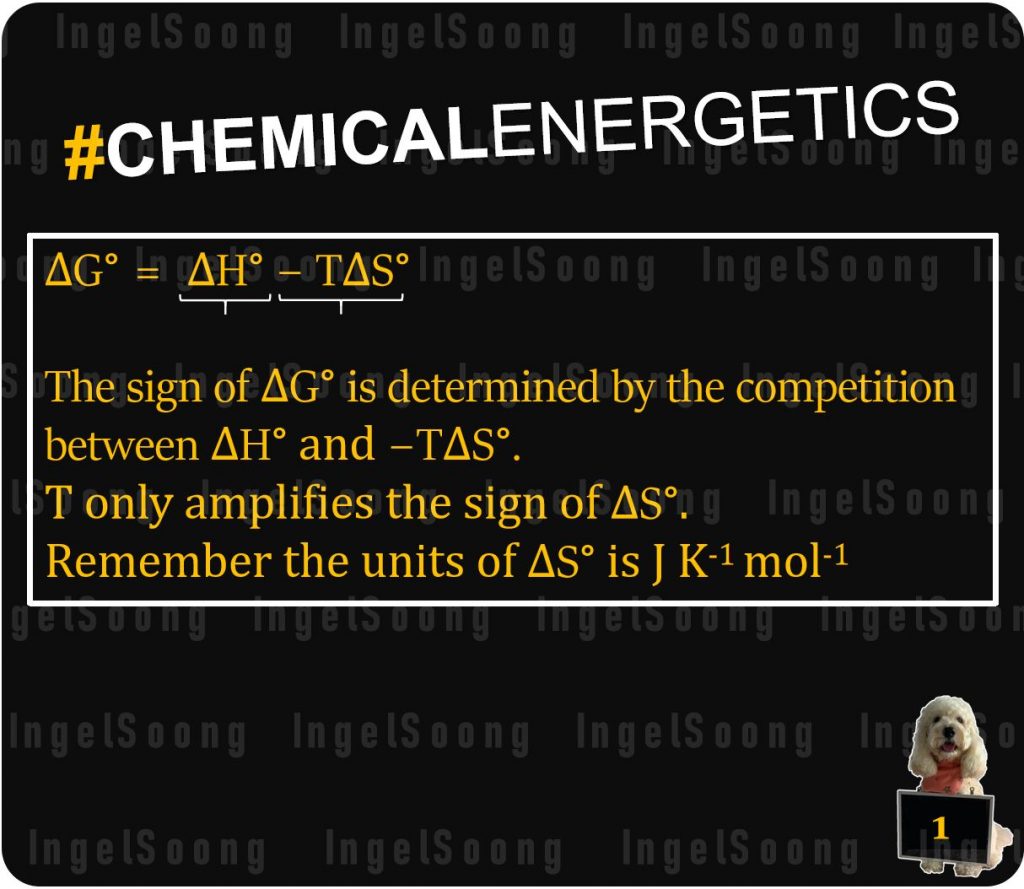
The sign of $\Delta G^o$ is determined by the competition between $\Delta H^o$ and -T\Delta S^o $.
Remember the units of $\Delta G^o$ is $ KJ mol^{-1} $ while $\Delta S^o$ is $ JK^{-1} mol^{-1} $