Mole Concept Redox Questions
Here’s an “ingelious” way to solve redox questions related to mole concept.
Let’s look at a typical JC A Level H2 Chemistry question:
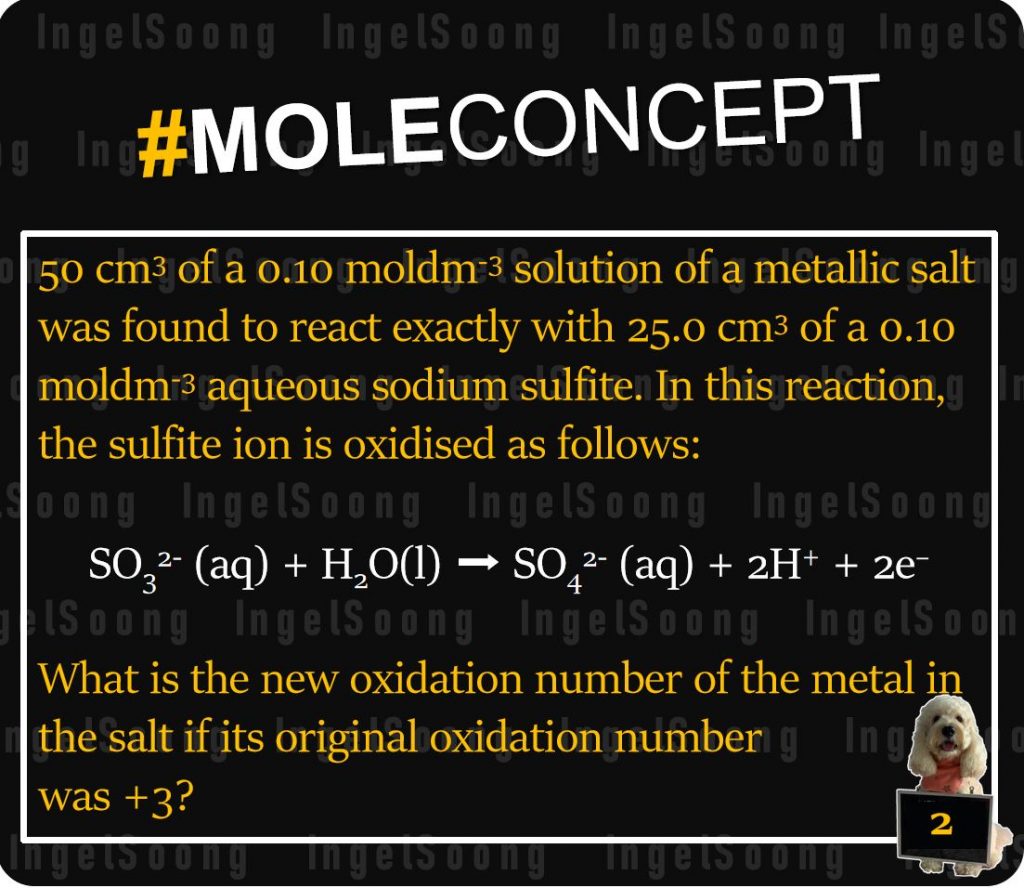
50 cm3 of a 0.10 moldm-3 solution of a metallic salt was found to react exactly with 25.0 cm3 of a 0.10 moldm-3 aqueous sodium sulfite. In this reaction, the sulfite ion is oxidised as follows:
SO32- (aq) + H2O(l) SO42- (aq) + 2H+ + 2e–
What is the new oxidation number of the metal in the salt if its original oxidation number was +3?
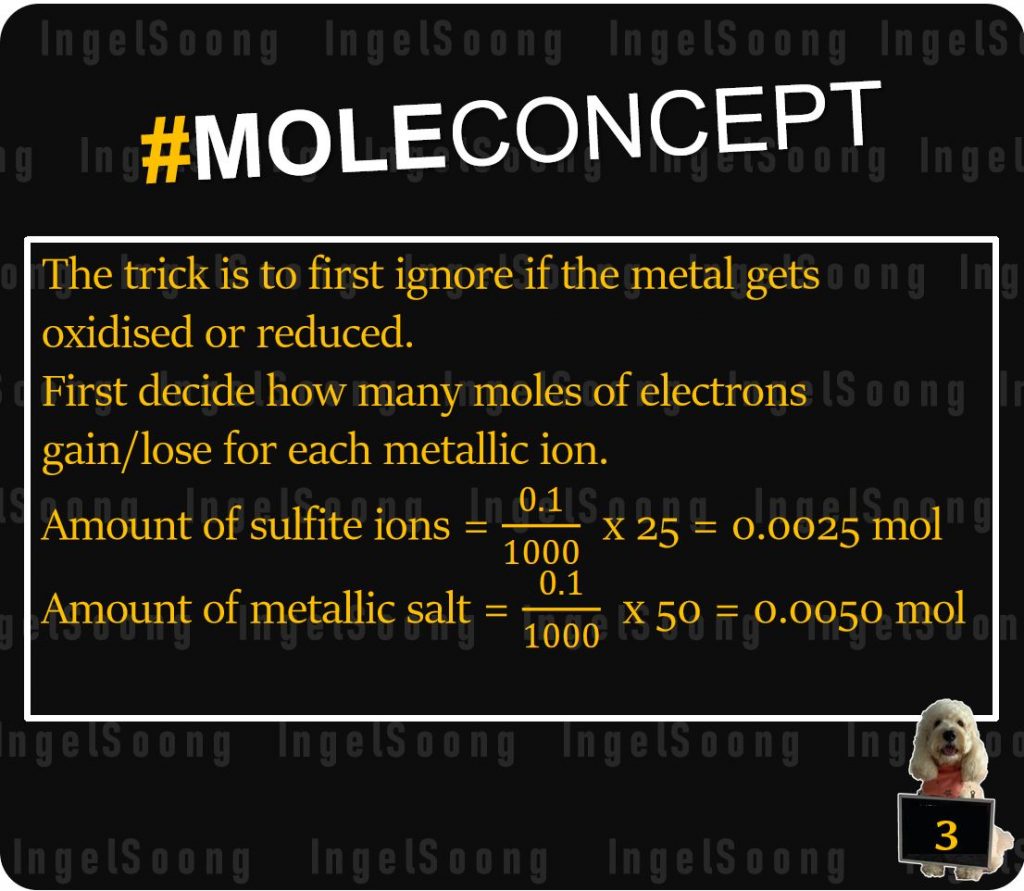
The trick is to first ignore if the metal gets oxidised or reduced.
First decide how many moles of electrons gain/lose for each metallic ion.
Amount of sulfite ions = x 25 = 0.0025 mol
Amount of metallic salt = x 50 = 0.0050 mol
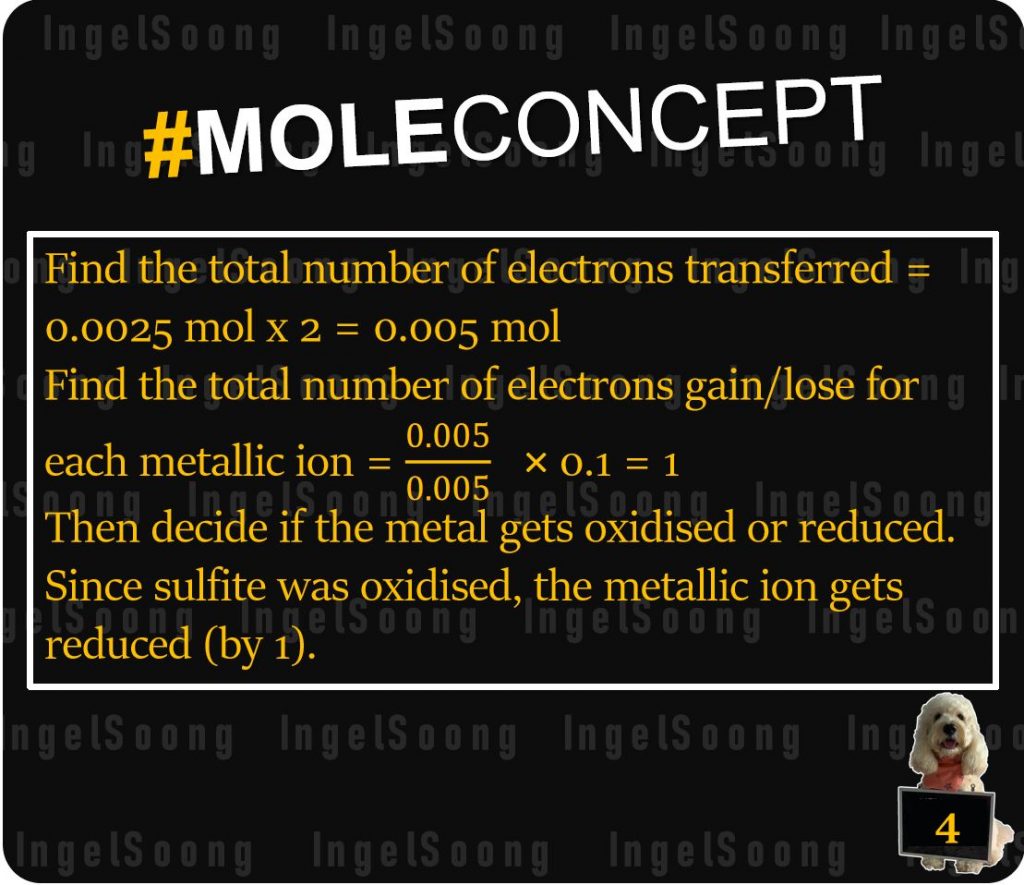
Find the total number of electrons transferred = 0.0025 mol x 2 = 0.005 mol
Find the total number of electrons gain/lose for each metallic ion = = 1
Then decide if the metal gets oxidised or reduced.
Since sulfite was oxidised, the metallic ion gets reduced (by 1).
As the original oxidation number of the metal was +3, the new oxidation number is now +2.
As an analogy, consider there are 25 boys in the class.
Each boy will pass \$2 to the girls in the class for a total of \$50 dollars.
If there are 50 girls in the class, each girl will receive \$1.
Since each girl has \$2 from the start, they will now have \$3 each.