Chemical Energetics Bond Energies
Here’s an “ingelious” way to solve bond energies questions related to chemical energetics.
Let’s look at a typical JC A Level H2 Chemistry question:
Find the enthalpy change of reaction for
Bond energy N-H 390 kJ mol -1
Bond energy F-F 158 kJ mol -1
Bond energy H-F 562 kJ mol -1
Bond energy N-F 272 kJ mol -1
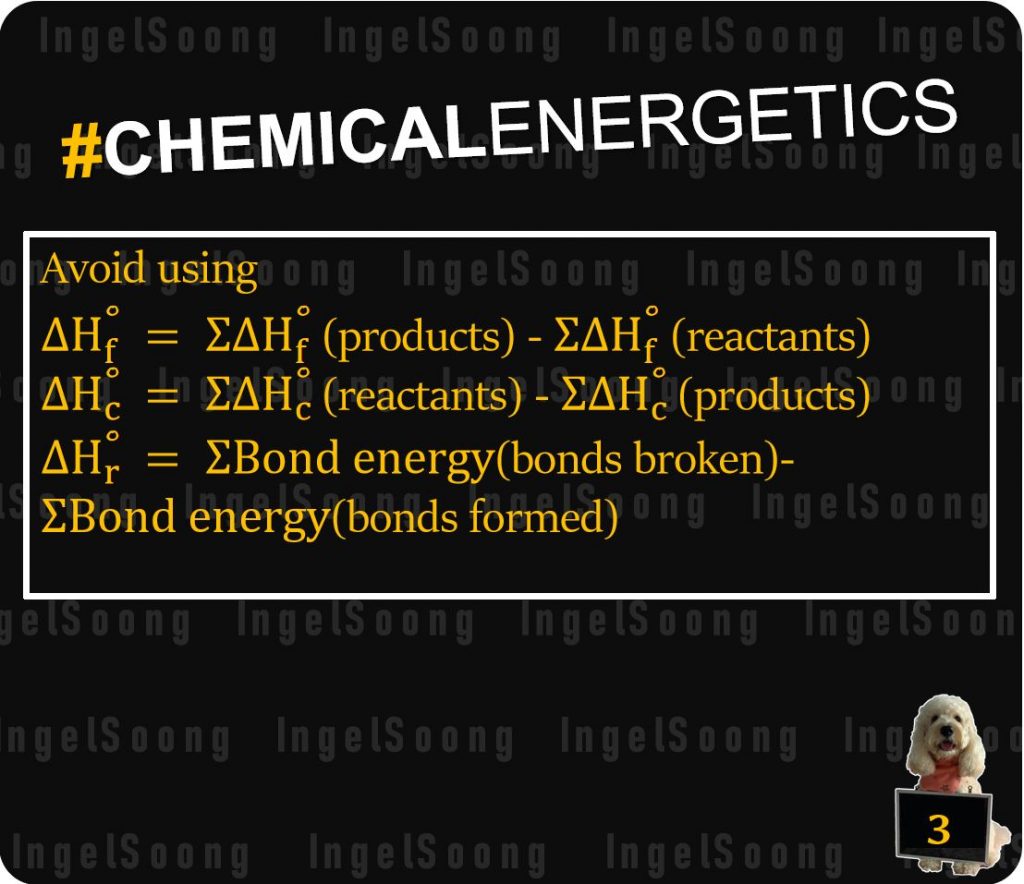
Avoid using
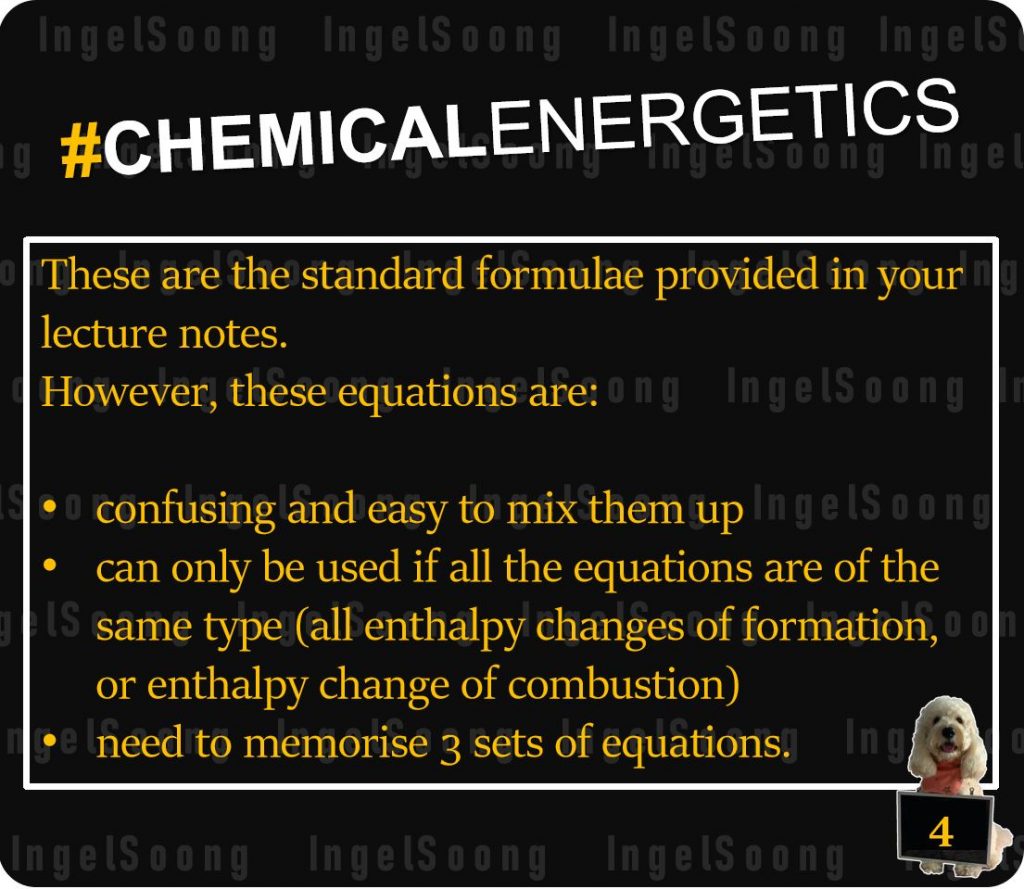
I know these are the standard formulae provided in your lecture notes.
However, these equations are:
- confusing and easy to mix them up
- can only be used if all the equations are of the same type (all enthalpy changes of formation, or enthalpy change of combustion)
- need to memorise 3 sets of equations.
Instead I will use the following method:
1) Consider the total energy taken in to break bonds and total energy given out to form bonds.
2) Take the difference in magnitude.
3) Then add “+” if the overall energy is endothermic or “-” if the overall enthalpy is exothermic. The idea is to decouple magnitude and sign convention.
Total energy absorbed = 3(390) + 3(158) = 1644 kJ mol -1
Total energy released = 3(272) + 3(562) = 2502 kJ mol -1
Overall difference = 2502 – 1644 = 858 kJ mol -1
Since total energy released > total energy absorbed, overall reaction is exothermic.